Biofiltration Fundamentals
Industrial Biofilter Processes
Fundamentals of Biofiltration
How Does Industrial Biofilter Work?
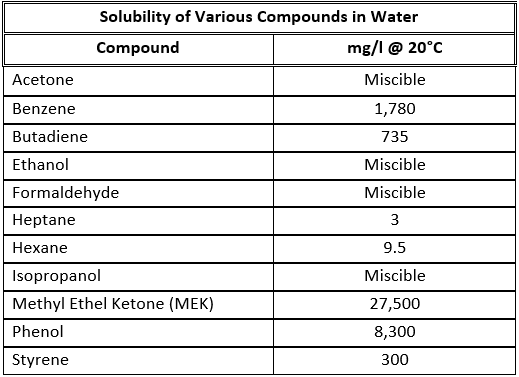
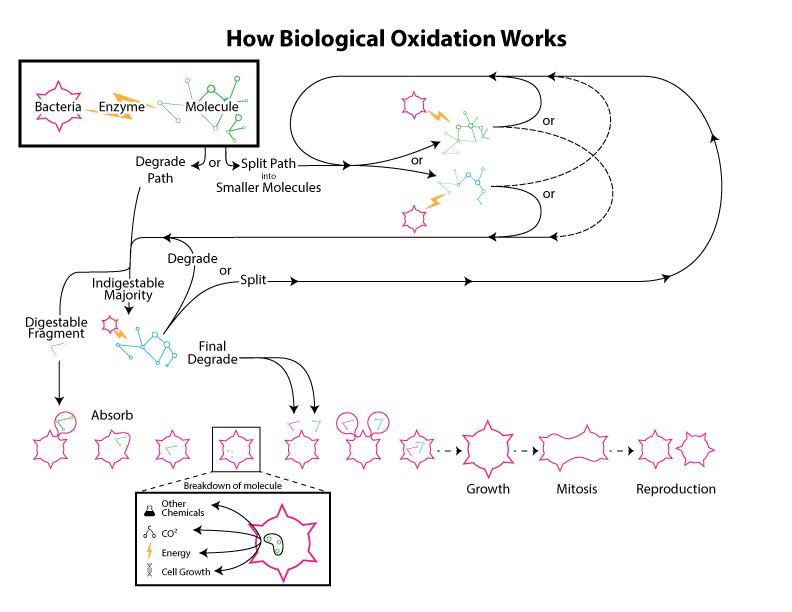
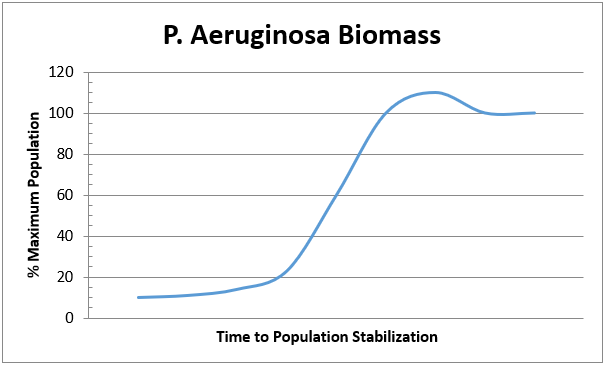
In the simplest terms, a biofilter is a device that utilizes natural biological oxidation for the destruction and / or removal of hydrocarbons (or CS2, H2S, NH3), that is to say biofiltration is the degradation of organic and inorganic substances by micro-organisms. These micro-organisms live in a biofilm coating that resides on the surface of a media composed of organic or inorganic (or a combination thereof) matter. The micro-organisms are for the stationary in regard to the system as a whole though they are mobile in their localized biofilm area. The process gases containing the contaminants to be treated flow through the media and as these gases flow by molecules of contaminants pass very near to or directly contact the biofilm where they are absorbed into the biofilm. Noting that the biofilm is primarily composed of water one can see clearly that a compounds solubility in water will greatly impact ease of degradation because if the compound does not enter the biofilm then it cannot be decomposed by the micro-organisms in that biofilm. The biofilm also creates a fixed (more or less) inhabitable space that can only be utilized by a finite maximum number of organisms. The organisms will grow and expand until the available space (biofilm) is filled resulting in situation where no more effective growth can occur.. This means that the effective amount of the summation of biomass (dead and alive) in the unit is at a relative constant. An example of this is shown in the graph below.
What can be degraded in a Biofilter?
Biofilters can be used to degrade many different kinds of compounds with a wide range of industries. Some of the compounds that can be degraded include:
- Acetone
- Aliphatic Hydrocarbons
- Ammonia
- Anthranilates
- Aromatic Hydrocarbons
- Butadiene
- Carbon Disulfide
- Esters
- Ethanol
- Ethers
- Formaldehyde
- Heptane
- Hexane
- Hydrogen Sulfide
- Isopropanol
- Isopropyl acetate
- Ketones
- Methyl Ethel Ketone (MEK)
- Methanol
- n-Propanol
- N-propyl acetate
- Pesticides
- Phenol
- Pinenes
- Styrene
- Terpenes
- VM&P naptha
How are compounds degraded?
Virtually any force that affects the biofilm can affect the operation of the biofilter. Factors that can affect the biofilm include: Moisture, temperature, residence time, particulate load, nutrient availability, pH and poisons.
On what processes are Biofilters used?
Biofilters have been employed on various processes and pieces of process equipment such as:
- Composting
- Door & Window Manufacturing
- Flavor & Fragrance Production
- Food Processing
- Frying Operations
- Medium Density Fiberboard (MDF)
- Paint Spray Booths
- Particleboard Manufacturing
- Pet Food Production
- Petro-Chemical Plants
- Pharmaceutical Prodution
- Photo and Film Production
- Printing
- Pulp & Paper Manufacturing
- Sewage Processing Plants
- Tanneries
- Textile Fabrication
- Waste Water Treatment Plants (WWTP)
What factors influence the sizing of Biofilters?
There are many factors that can affect the correct sizing of a biofilter. Some characteristics such as a compound's solubility in water have a greater impact than others. Some factors include:
- A compound's Solubility in water
- A compound's Concentration
- A compound's Henry's Law Constant
- A compound's Diffusion rate
- Temperature of the flue gas
- Media density
- Media porosity
How is the size of a Biofilter determined?
Each type of compound or gas has a different potential of being utilized by the bacteria as food and as such an imperial model is generated from test reaction and diffusion data gathered in lab scale, pilot scale and full scale applications. Once the model is constructed a biofilter's media volume can be determined by entering the concentration of each known compound in the gas stream and not just the concentrations of the compounds to be degraded because bacteria will eat the most easily digested molecules first. As a general rule the more insoluble a compound is the less biodegradable that compound is, or to put it another way the less soluble a compound is more media, and thus bacteria, it will take to degrade that compound.